Basics of BiologyBasic Chemistry For Biology |
How does one prepare a 1:10 dilution? |
To dilute means to weaken or reduce the intensity, strength, or purity of a substance, or to make more fluid by adding a liquid. For example, a 1:10 dilution means one part in a total of ten parts. Three different ways exist to prepare a 1:10 dilution: 1) the weight-to-weight (w:w) method, 2) the weight-to-volume (w:v) method, and 3) the volume-to-volume (v:v) method. In the weight-to-weight method, 1.0 gram of a solute (a substance dissolved in a solution or mixture of some type) is dissolved in 9.0 grams of solvent (a substance having the ability to dissolve another substance), yielding a total of ten parts by weight, one of which is solute. In the weight-to-volume method, enough solvent is added to 1.0 gram of solute to make a total volume of 10 millileters. In this method, one part (by weight) is dispersed in ten total parts (by volume).
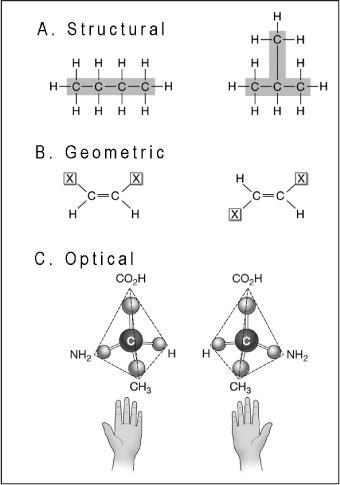
The three types of isomers are A) structural, which are connected in different ways. In this example, butane and isobutane (called an isomer of butane) differ in covalent partners; B) geometric, which differ in arrangement about a double bond (in these diagrams X represents an atom or group of atoms attached to a double-bonded carbon; and C) optical (or enantiomers), which are mirror images of each other, like left and right hands—but they cannot be superimposed on each other.
