Basics of BiologyBiology and Life |
Water is the universal solvent in biological systems, so what does this mean for living organisms? |
A solvent is a substance that can dissolve other matter; because all chemical reactions that support life occur in water, water is known as the universal solvent. In fact, it is the polar nature of the water molecule (it contains both positive and negative poles) that causes it to act as a solvent—and any substance with an electric charge will be attracted to one end of the molecule. (If a molecule is attracted to water, it is termed hydrophilic; if it is repelled by water, it is termed hydrophobic.)
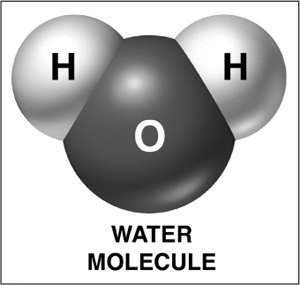
A water molecule is essential to life on Earth. Its slightly positive and negative poles encourages other molecules to organize themselves in aqueous solutions.
