Organic ChemistryReactions of Organic Compounds |
Are there uni- and bimolecular elimination reaction mechanisms, like there were for substitution reactions? |
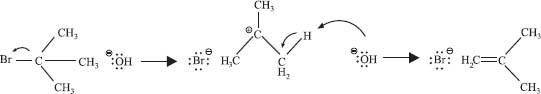
Yes! And what do you think controls whether an elimination reaction happens in one step or two? Right—the stability of the carbocation. In the previous example, if bromide ion had dissociated first, a primary carbocation would have formed:
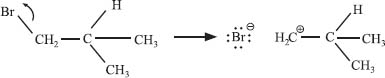
This is a much more difficult reaction than the bimolecular process where the elimination of HBr takes place in a single step.
But if an alkyl halide can form a stable carbocation, the unimolecular elimination reaction is faster. It’s referred to as “unimolecular” because the slow step has only one molecule in the transition state, just like substitution reactions.