Atoms and MoleculesProperties of Atoms and Electrons in Atoms |
How do the atomic radii of atoms change across the table? |
The atomic radii of atoms generally decrease going from left to right across a period, and increase going top to bottom down a group (see graphic on next page).
The increase in atomic radius going down a group is fairly straightforward to understand: additional shells of electrons are added and they must surround the inner shells, resulting in an increased atomic radius. Though the number of protons in the nucleus increases going down a group, the inner shells of electrons serve to shield the valence shell from the attractive force of the nucleus, resulting in an overall increase in atomic radius.
Moving to the right across a period, the number of protons increases, increasing the attractive force on electrons in the valence shell. Within a period, additional electrons go into the same valence shell, and an increasing attractive pull from the nucleus results in a more contracted valence shell, resulting in a smaller atomic radius. The situation is complicated by the rightmost group (known as the Noble gases), but the atomic radius of these elements is typically not important as they are rarely involved in chemical bonds to other atoms.
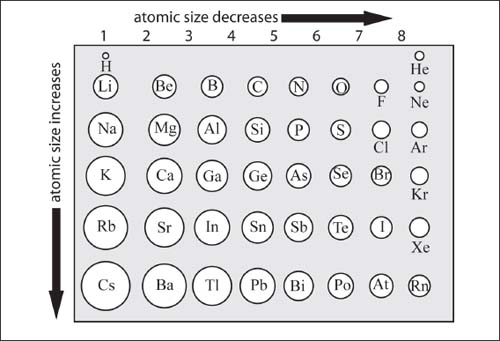
The atomic radii of atoms generally decrease going from left to right across a period, and increase going top to bottom down a group. (Atomic sizes are not to precise ratios and are for illustrative purposes only.)
