Organic ChemistryStructures and Nomenclature |
What are diastereomers? |
This is going to sound like a cop-out, but diastereomers are stereoisomers that are not enantiomers. That’s the real, technical definition. One type of diastereomers show up when carbon forms a double bond. Recall from previous chapters that when there are three groups bonded to a carbon atom, it will be planar (sp2 hybridized). If the double bond is in the middle of a carbon chain, there are two possible isomers.
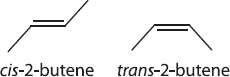
These two molecules are not superimposable, but they’re also not mirror images, so they are called diastereomers. There are many other forms of diastereoisomers, but this form is the easiest to understand.
