Atoms and MoleculesMolecules and Chemical Bonds |
What are some common structures/geometries for molecules? |
The study of chemistry has benefited greatly from knowledge of properties relating to the geometries and, especially, the symmetries of molecules. To get a sense of what shapes molecules adopt, it’s worth taking a look at a few of the geometries that come up often in the study of chemistry.
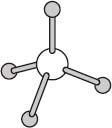
One commonly encountered geometry is that of a tetrahedron. Methane has the molecular formula CH4 and exists in a tetrahedral geometry with angles of approximately 109 degrees between each pair of C–H bonds.
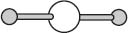
Linear geometries are also relatively common. Carbon dioxide has the molecular formula CO2 and exists in a linear geometry with a 180-degree angle between the CO bonds.
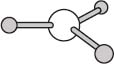
One last geometry we’ll look at here is a planar geometry. The molecule BH3 provides one example of a planar geometry, and in this case the BH bonds are separated by angles of 120 degrees. There are also planar molecules with four bonds in a plane, and in those cases the bonds are separated by angles of 90 degrees.
