Atoms and MoleculesMolecules and Chemical Bonds |
What is Coulomb’s law? |
Coulomb’s Law tells us the force experienced by a pair of separated charges. It’s a fundamental equation in the study of electrostatics, which is a broad area of physics concerned with the interactions between stationary charges. The equation for this force can be written:
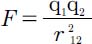
where charges q1 and q2 are separated by a distance r12 and have a “unit of charge” defined by:
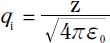
in which z is the charge in Coulomb’s and ε0 is the permittivity of free space, a fundamental physical constant.
The key features of Coulomb’s Law are that it predicts an attractive force between particles of opposite charge and that this force decreases with the square of the distance between the particles. For chemistry, it’s relevant to point out that the force between charges falls off rather slowly with the distance between them, so where charges are present in relatively dense materials (like liquids and solids), they have a significant effect on their environment.
