Chemical ReactionsKinetics and Thermodynamics |
What is Le Chatelier’s principle? |
Le Chatelier’s principle tells us how to predict the effect a change in conditions will have on a chemical equilibrium. It tells us that a system at equilibrium will shift to counteract changes that disturb the equilibrium. These could be changes in concentrations of chemical species, temperature, pressure, or other conditions. The most commonly discussed changes involve changes in concentration of chemical species, so we’ll just focus on those here. For this equilibrium:
If we decrease the concentration of A, some C and D will react to replenish the A that is depleted, so the concentrations of C and D will decrease. As species A is replenished, more B will be created as well. So the net effect is that decreasing the concentration of A will also decrease the concentrations of C and D, and at the same time increase the concentration of B. More generally, decreasing the concentration of a reactant will cause the equilibrium to shift toward the reactants, increasing the concentrations of other reactants and decreasing the concentrations of products. The converse is also true: Decreasing the concentration of a product will cause the equilibrium to shift toward the products, increasing the concentrations of other products and decreasing the concentrations of reactants.
It is important to keep in mind that Le Chatelier’s principle only applies to reversible chemical processes (chemical equilibria), so everything we have said here does not apply to reactions that can only proceed in the forward direction.
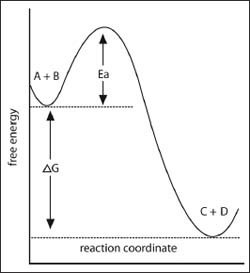
An example of a free energy diagram.
