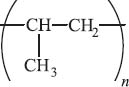
If instead of substituting a chlorine atom we add a methyl group to each ethylene monomer, we get polypropylene. Recall from earlier that this introduces stereochemistry along the polymer. We’ve already mentioned that the arrangement of the methyl groups along the polymer chain can have large effects on the melting point and other physical properties. You can likely find polypropylene all over your house from dishwasher-safe food containers to synthetic carpets (especially outdoor carpeting) and an increasing weight fraction of your car, including the bumper and the casing for the battery. It can also be made into ropes, which are quite strong and resistant to weather, so they are frequently used in fishing and farming. Polypropylene is also used for many medical applications because it is capable of withstanding the high temperatures required to sterilize.