Atoms and MoleculesMolecules and Chemical Bonds |
What is a Lewis structure? |
Lewis structures are a simple way of depicting the electronic structure of atoms and molecules. They show us which atoms are bonded to each other in a molecule and also show how many nonbonded electrons are present in the valence electron shell of each atom. The easiest way to understand them is probably to just take a look at a few.
The simplest Lewis structure is that for a single hydrogen atom. It has just one electron, and its Lewis structure looks like this:
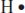
The letter H lets us know that it’s a hydrogen atom, and the one dot represents its one electron.
Moving on to the Lewis structure for a molecule, let’s look at the Lewis structure for F2:
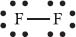
Here the two Fs let us know there are two fluorine atoms. The line connecting them shows that they are bonded with a single bond (containing two electrons). Each has six more electrons surrounding it, and these electrons are nonbonding.
And finally for a molecule with more than one bond, CH2O:
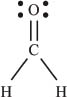
This molecule is called formaldehyde. The Lewis structure shows us that the carbon is involved in a single bond (sharing two electrons) with each hydrogen atom, and a double bond (sharing four electrons) with the oxygen atom. The oxygen atom also has four nonbonding electrons.
