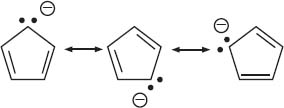
These are molecules with bonds between carbon and a metal. This includes alkali and alkali earth metals (Groups 1 and 2 of the periodic table), the transition metals (Groups 3–12, including the f block), and sometimes Group 13 metals are also included. The bonds between metal and carbon can vary widely in their ionic or covalent character. The bonding between a metal and carbon is mostly ionic in two situations: (1) if the metal is very electropositive, as in Groups 1 and 2; or (2) if the carbon group is a stable anion (by being delocalized via resonance as in cyclopentadienyl anion). There are also organometallic molecules with more covalent bonds between the metal atom and the carbon atom. This is usually seen with the transition metals or elements like aluminum. The nature of the metal-carbon bond plays an important role in how organometallic complexes react. The resonance structures of the cyclopentadienyl anion are below: