Organic ChemistryReactions of Organic Compounds |
What is hyperconjugation? |
Hyperconjugation is another way to explain why substituted carbocations are more stable. The electrons in either the C–C or C–H bonds that are near the cationic center can interact with the empty p-orbital. It’s not the bonds that are directly connected, but rather one bond removed from the center carbon, that are the most important (see the illustration below). At the simplest level, this provides an explanation for the statement in the previous answer that neighboring carbon atoms are more electron-donating than hydrogen atoms: it’s really the neighboring C–H bonds that help stabilize the empty p-orbital.
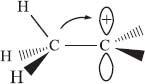
Note: The arrow above is just to illustrate the overlap of the C–H σ bond with the empty p orbital. It’s not to suggest that the hydrogen atom actually moves…though this does happen sometimes!
