Polymer ChemistryPolymers in and Around You |
What is nylon? |
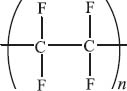
Nylon is a synthetic polymer formed by the condensation of a dicarboxylic acid with a diamine. This reaction forms an amide bond and releases a molecule of water. The term “nylon” is a generic name for these types of polymers, but one of the common nylons is called “Nylon 66.” The numbers signify the number of carbon atoms in the amine (6) and the acid (6) reactants.