Physical and Theoretical ChemistryEnergy Is Everything |
What is osmosis? |
Osmosis is the movement of solvent molecules in a solution to establish an equal concentration of solute throughout the solution. Solvent molecules move from areas of low-solute concentration to areas of high-solute concentration, which tends to remove any gradient in solute concentration.
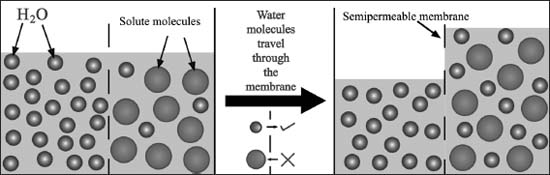
In osmosis, solvent molecules move from areas of low solute concentration to areas of high solute concentration through a permeable membrane to equalize the solute concentration on either side.
