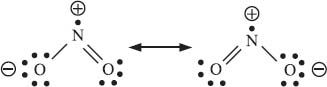
Resonance is a way that chemists represent delocalized electronic structure. Let’s take that statement apart to understand what it means. “Delocalized” means that an electron, or a pair of electrons, is not located entirely around a single atom or bond. Take a look at the two structures of nitrogen dioxide (NO2) on the following page. The negative charge is located on one oxygen atom in one resonance structure, but can be found on the other oxygen in the second resonance structure. Notice that we said “electronic structure” and haven’t said anything about atoms moving here—that’s because they don’t. Resonance only deals with electrons, and the atoms are located in the same arrangement in every resonance contributor. This is important, and makes sense if you remember that the electrons aren’t “moving” from one resonance structure to another. These structures are needed because the actual molecule is more complicated than our simple drawing system can represent.