Inorganic ChemistryElectricity and Magnetism |
What is the difference between paramagnetic and diamagnetic complexes? |
In chemistry, atoms or molecules that have at least one unpaired electron (so there is a net spin to the molecule) are known as paramagnetic. If all electrons are paired, chemists refer to the compound as diamagnetic. When a magnetic field is applied a paramagnetic substance will be attracted to the field, while diamagnetic molecules will be repelled from the field.
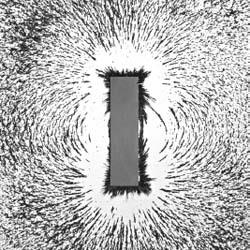
The iron shavings surrounding this magnet provide a good idea of the shape of the magnetic field surrounding this bar magnet, including its north and south poles.
