Organic ChemistryStructures and Nomenclature |
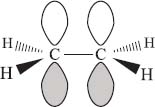
What is the shape of a carbon-carbon double bond? |
The geometry of the carbon atoms in double bonds is planar. This shape comes from the hybridization of the carbon atom, which is sp2 (one p orbital is not involved in forming single bonds). To get a bonding interaction between these two remaining p-orbitals, they have to overlap in space. So in a molecule like ethylene (C2H4), all of the hydrogen atoms are located in the same plane.