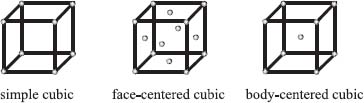
There are three basic packing arrangements, which we can describe by again imagining a tiny box. If we place an atom at each of the eight vertices of this box, the arrangement is referred to as simple cubic. If we take a simple cubic unit cell and add an atom to the center of each face, we have a face-centered cubic arrangement. If we instead add an atom to the center of the cube, it’s called a body-centered cubic unit cell. There are more possibilities, but these three simple ones described here cover a lot of the crystals that chemists encounter.