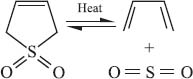
Finally, there’s a second type of reaction that involves two π-bonds being transformed into two σ-bonds. The difference here is that two bonds are formed (or broken) at a single atom, while in cycloaddition reactions, only one bond is made at each reactive atom. An example involving SO2 is shown below (surprisingly this reaction isn’t named after anyone!). In this reaction both σ-bonds are made (or broken) at the sulfur atom. Compare this with the Diels-Alder reaction mentioned above, where the two new σ-bonds are connected to the two different ends of the dieneophile.