Organic ChemistryReactions of Organic Compounds |
What’s an electrocyclic reaction? |
Okay, so a cycloaddition reaction was 2π 2σ, and we just saw that sigmatropic reactions are 1σ
2σ, and we just saw that sigmatropic reactions are 1σ 1σ, so what about a pericyclic reaction that is 1π
1σ, so what about a pericyclic reaction that is 1π 1σ? That’s an electrocyclic reaction. Like most pericylic reactions, electrocyclic reactions can either make or break a σ-bond. If a σ-bond is made in the process, it’s an electrocyclic ring-closing reaction. If the σ-bond is consumed in the process, the process is named an electrocyclic ring-opening reaction, but it’s the same process.
1σ? That’s an electrocyclic reaction. Like most pericylic reactions, electrocyclic reactions can either make or break a σ-bond. If a σ-bond is made in the process, it’s an electrocyclic ring-closing reaction. If the σ-bond is consumed in the process, the process is named an electrocyclic ring-opening reaction, but it’s the same process.
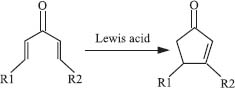
One noteworthy example of an electrocyclic ring-closing reaction is the Nazarov cyclization, which converts divinyl ketones into cyclopentenes, usually in the presence of an acid.