MacRoscopic Properties: The World We SeePhases of Matter and Intensive Properties |
Why does ice float? |
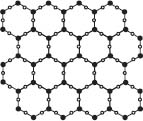
Ice floats in water because it is less dense than water, though this is actually a very unusual case in terms of comparing the densities of the solid and liquid phases for a given substance. Most substances increase in density when moving from the liquid to the solid phase of matter, but H2O does the opposite. When water freezes, it forms a network of hydrogen bonds between H2O molecules, and because of the spacing of the molecules in this lattice, ice is less dense and floats in water.