Math in the Physical SciencesChemistry and Math |
What is an electron? |
Chemically speaking, matter is composed of minute particles called atoms; in turn, atoms contain elementary (or subatomic) particles: protons (positively charged particles), neutrons (particles with no charge), and electrons (negatively charged found around the nucleus of the atom). When an atom gains or loses electrons, it acquires a net electric charge.
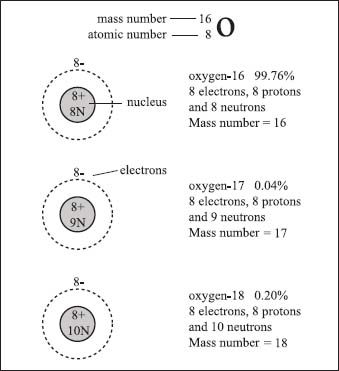
Three naturally occurring isotopes of oxygen are oxygen-16, oxygen-17, and oxygen-18. They differ by the number of neutrons in their nuclei. Oxygen-16 is the most common form of oxygen in our atmosphere at 99.76% occurrence.
There is a strange part of an electron’s definition, especially in the field of physics: is an electron a particle or a wave? In reality, everything is a wave or particle; and the more mass an object has, the smaller the waves. Thus, electrons, which have less mass, have larger waves. In quantum mechanics, electron waves are actually thought of as probability waves (and so are matter waves in general). But again, there are some different characteristics of these waves. In particular, the wave does not indicate where the electron is found, only where it might be found—or the probability of the electron being in that place.
