Math in the Physical SciencesChemistry and Math |
What is the pH scale? |
The “pH” scale stands for p(otential of) H(ydrogen) scale, or the logarithm of the reciprocal of hydrogen-ion concentration in gram atoms per liter. In simpler terms, the pH is merely the measure of the hydrogen ion concentration of a solution. The pH numbers are based on a scale from 0 to 14, in which numbers less than 7 represent acidic solutions and numbers greater than 7 represent alkaline (base) solutions. A reading of 7 is considered neutral.
Mathematically speaking, once the concentration of hydrogen ions is determined chemically (based on moles per liter), the pH value is established by taking the exponent used in expressing this concentration and reversing its sign. It is most often expressed as the notation pH = -log 10[H+]. For example, if the hydrogen ion concentration of a solution is determined to be 10-4 (or 0.0001) moles per liter, the pH is 4.
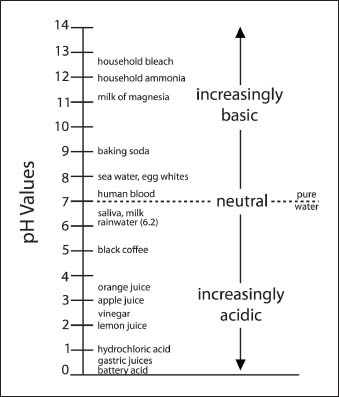
A pH scale indicating pH levels for common liquids and other substances.
Many people are familiar with the pH scale from high school, especially the practice of using special whitish paper called litmus paper to check for pH. The paper contains a powder extracted from certain plants, allowing the user to determine acidity (the paper turns red), neutrality (the paper stays white), or alkalinity (the paper turns blue) of various solutions. The stronger the acid or base, the more intense the red or blue, respectively. And pH isn’t just for use in chemistry class. For example, it is also important to people who work the soil. All plants need a certain soil pH to grow and flourish, which is why most gardeners and farmers determine the acidity or alkalinity of their soil in order to grow better crops.
