Thermal PhysicsThermodynamics |
How do refrigerators and air conditioners work? |
As you know, when a liquid evaporates into a gas, it is cooled. Heat flows into the system. The opposite process, the condensation of a gas into a liquid results in an increase in thermal energy and an output of heat. A refrigerator circulates a refrigerant, a liquid that evaporates at a low temperature, through tubing, The gas is compressed by an electrically-driven compressor. The pressure and temperature of the gas increases. Coils of the tubing outside the refrigerator cool the liquid and heat the air around them. As it cools the refrigerant condenses back to a liquid that goes through a tiny hole, called the expansion valve. The pressure drops, evaporating the liquid, making the gas cold. The tubing containing the cold gas is in the inside of the refrigerator, making it cold, and cooling the food.
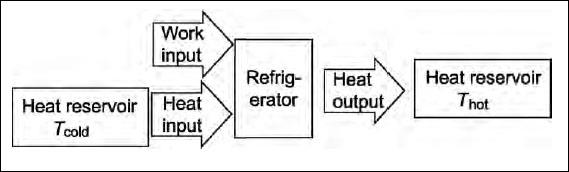
An air conditioner works in a similar way. The evaporator is in the unit inside the house and the compressor is outside. Because of the work put into the compressor heat is removed from the air inside the house and transferred to the outside air. The diagram below shows work and heat flows in a refrigerator or air conditioner.
The first home refrigerators used ammonia as a refrigerant, but ammonia is toxic. In the 1930s Freon was first developed by the DuPont Company of Wilmington, Delaware. Freon is a chlorofluorocarbon (CFC). If Freon escapes it carries chlorine atoms to the upper atmosphere. There ultraviolet radiation from the sun separates one of the chlorine atoms from the CFC. That atom converts ozone back to oxygen, contributing to the destruction of the ozone layer, an essential barrier against harmful ultraviolet sunlight.
Freon’s destructive nature has been known since the 1970s, but it was not until the early 1990s that legislation was implemented banning the use of Freon in new air conditioners and refrigerators. It has been estimated that in 2002 there was six million tons of Freon in existing products. Unfortunately, when the chlorine destroys an ozone molecule, the chlorine is not destroyed, but instead continues to live for a while destroying more ozone. In fact, more Freon is still headed toward the upper limits of the atmosphere, for it can take several years for Freon to reach such elevations.
DuPont and other corporations have developed replacements for Freon that replace the chlorine atoms with hydrogen atoms. These substances do not harm the ozone layer and are in use in refrigerators, air conditioners, and aerosol cans.