What Is the World Made Of?Introduction |
What is the structure of the atom? |
Thomson pictured the atom as a swarm of electrons in a positively charged sphere. This model is called the “plum pudding” model, after a then favorite English Christmas treat. Americans might picture a ball of pudding filled with raisins.
The New Zealand born physicist Ernest Rutherford (1871-1937) developed a method of testing Thomson’s model. Rutherford’s first scientific work was done in Montréal, Canada beginning in 1898. He and Frederick Soddy conducted a study of radioactivity and radioactive materials that won Rutherford the 1908 Nobel Prize in Chemistry. Those experiments will be described in the chapter on nuclear physics, “At the Heart of the Atom.” He studied the radiation emitted by thorium and uranium, which he named alpha and beta rays. He recognized that alpha rays would be ideal probes for studying materials.
Rutherford moved to the University of Manchester in Britain in 1907 where he worked with Hans Geiger (1882-1945; later to invent the Geiger counter) on ways of detecting individual alphas. They determined that the alpha particles were doubly charged. They allowed the alphas to go through a window made of very thin mica (a mineral that could be cut into very thin slices). He noticed that the rays that penetrated the mica were deflected slightly more than they should have been by a plum-pudding atom. With Geiger and Ernest Marsden, he directed a beam of alphas on extremely thin foils of gold. To their great surprise they found a significant number of alphas were deflected into very wide angles—some greater than 90°. Rutherford commented that it was as if a 15” shell from a naval cannon bounced off a sheet of tissue paper.
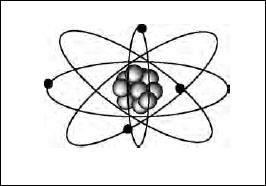
By 1911 Rutherford had developed his own model of an atom. It consisted of an extremely small positively-charged particle (later called the nucleus) surrounded by electrons. Although the paths of the electrons were not mentioned in the 1911 paper, it was later assumed that they orbited the nucleus as planets orbit the sun. The nucleus was 1/10,000 as large as the atom, but had all the positive charge and essentially all of its mass. If the atom’s mass was equal to N hydrogen masses, then the nuclear charge was about N/2. The electrons carry a total charge equal and opposite that of the nucleus so that the atom would be electrically neutral. Rutherford’s atom is mostly empty space!