Organic ChemistryStructures and Nomenclature |
Are hydrocarbons always straight chains of carbon atoms? |
No. There could be carbon atoms attached to the linear chains we talked about in the previous question. Let’s learn the next step in naming alkanes and have a look.
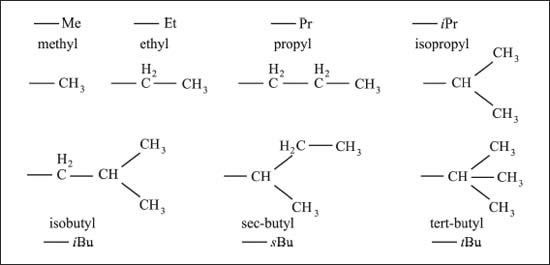
First, we need to define the names of branches (see graphic, next page). Chemists use the same prefixes to indicate the length of the branch, but now the suffix is “-yl” instead of “-ane.” So methane (CH4), when it’s a branch off of a chain of carbon atoms, becomes methyl (-CH3), ethane (CH3CH3) becomes ethyl (-CH2CH3), and so on.
Next we have to indicate where along the main carbon chain the branch point is. This part is pretty simple—just number the carbon atoms and put this number before the name of the branch. So if you had an eight-carbon chain (octane) with a two-carbon branch (ethyloctane) on the third carbon from the end, it would be called 3-ethyloctane and look like this:
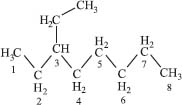
There are a lot more rules to naming organic compounds, but that’s enough for now.
Hydrocarbons with additional atoms attached to linear chains have suffixes ending in “-yl” instead of “-ane.” Here are some examples.
