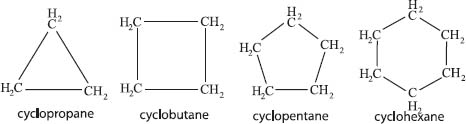
Yes—chains of carbon atoms can connect back to themselves, forming rings of atoms. The prefix cyclo- is added to the name of the linear carbon chain to indicate that a ring is present (so hexane becomes cyclohexane). The chemistry ring structures can be different than their linear cousins because of the added energy that some rings contain. We know that sp3-hybridized atoms like to form bonds that are separated by 109.5°. The more that a ring forces those bonds to deviate from that ideal angle, the more energy (called ring strain) that is released when that ring is opened during a chemical reaction.