Organic ChemistryReactions of Organic Compounds |
What is a bimolecular substitution reaction? |
From the last answer, you can probably guess that a bimolecular substitution reaction has two (bi-) molecules (-molecular) in the transition state. This means that the slowest step involves two molecules interacting. In the previous example, there was only one.
Let’s look at a similar substitution reaction, but instead of tBuCl, we’ll react hydroxide with MeCl:
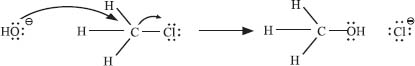
Here the nucleophile (OH−) directly displaces the leaving group (Cl−), without forming a carbocation intermediate. This is because the methyl cation (CH3+) is much less stable than the tert–butyl carbocation formed in the previous question.
