Organic ChemistryReactions of Organic Compounds |
What is a unimolecular substitution reaction? |
We’ve already talked about substitution reactions, so what makes one “unimolecular”? If the transition state (remember, this is the highest energy state of an individual chemical reaction) involves one (uni-) molecule (-molecular), then it is referred to as a uni-molecular reaction. This might seem like an odd thing to distinguish, but there are many differences between uni- and bimolecular substitution reactions. These differences all result from how many species are involved in the transition state.
For an example, here’s the reaction of tert–butyl chloride with hydroxide ion:
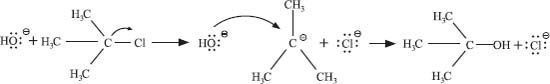
The first step is breaking the C–Cl bond, and this involves only the (CH3)3C–Cl molecule. Then the hydroxide ion reacts with the tert–butyl carbocation in the second step. Since there is only one molecule in the slower first step (just assume that’s true so we can illustrate this point), this is a unimolecular substitution reaction.
